Chemical formulas often become trending search topics as students, researchers, and curious learners explore organic chemistry concepts. One formula gaining attention recently is HCOOCH CH2 H2O, a combination that appears in discussions about organic reactions, hydrolysis processes, and formate-related compounds. Even though the formula looks unusual at first glance, it connects to several important chemical principles such as ester breakdown, functional groups, hydration, and structural analysis. This detailed, SEO-friendly article explains everything about HCOOCH CH2 H2O—its meaning, possible structures, reaction patterns, applications, and relevance in modern chemistry.
Understanding the Formula HCOOCH CH2 H2O
At first glance, the formula appears compact, but it represents meaningful building blocks in organic chemistry.
Breaking Down the Components
- HCOO– resembles a formate group
- CH2 often represents a carbon bridge or methylene unit
- H2O represents water, a molecule involved in countless reactions
When combined, this formula hints at a structure or reaction involving an organic compound undergoing transformation in the presence of water.
Why the Formula Is Frequently Searched
Chemistry students and researchers often look up formulas when:
- Studying organic reaction mechanisms
- Solving homework or exam questions
- Understanding hydrolysis
- Exploring functional groups
- Identifying unknown compounds
HCOOCH CH2 H2O appears frequently in structure-based learning tasks.
Possible Interpretation of HCOOCH CH2 H2O
Because the formula is simplified, chemists interpret it as a representation of an ester-like structure interacting with water.
Interpreting the Structure
Component Meaning
- HCOOCH could represent a formate ester
- CH2 could be a methylene unit linking groups
- H2O is likely part of a reaction rather than the molecular structure
This suggests the formula may describe a reaction, not a single molecule.
Most Likely Context: Ester Hydrolysis
In organic chemistry, a common reaction is ester + water → acid + alcohol.
Why This Matters
Esters break down in the presence of water through hydrolysis, especially under:
- Acidic conditions
- Basic conditions
- Heat
General Hydrolysis Reaction
Ester + Water → Alcohol + Carboxylic Acid
This aligns well with the formula’s components.
Breaking Down HCOOCH CH2 H2O in Hydrolysis Terms
HCOO– Group
Represents
Formate, the simplest carboxylate group.
CH2 Group
Function
Acts as part of the organic backbone or linkage.
H2O
Role
Starts the hydrolysis reaction by attacking the ester carbon.
Possible Reaction Example
One interpretation of the formula could be:
HCOO–CH₂ + H₂O → HCOOH + CH₂OH
Meaning
- Formate ester reacts with water
- Produces formic acid and an alcohol
Why This Reaction Is Important in Chemistry
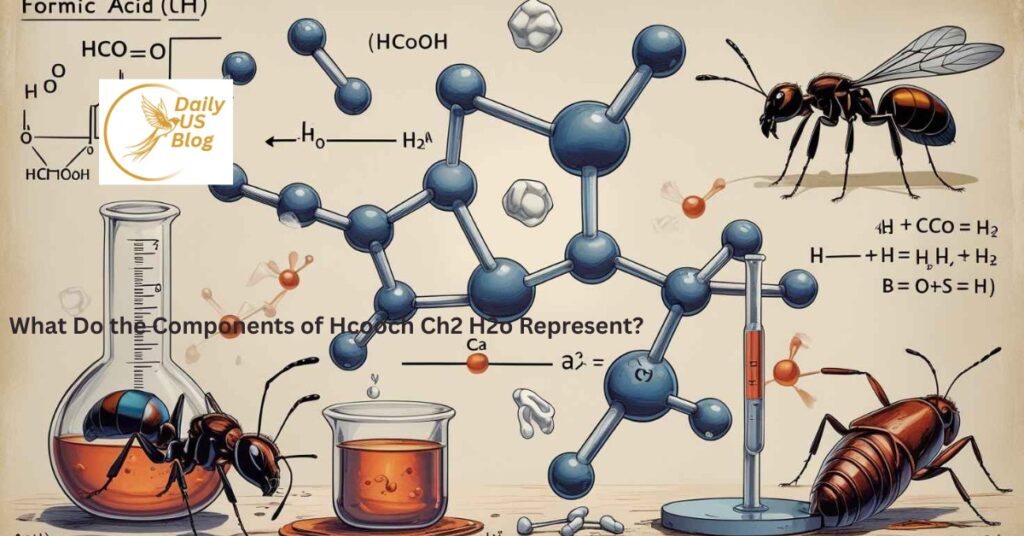
Hydrolysis reactions help explain how:
- Esters break down
- Biomolecules degrade
- Organic compounds transform in water
- Industrial processes produce alcohols and acids
Understanding Formate Esters
Formate esters are derived from formic acid.
Structure Overview
General Formula
HCOOR
Where R can be:
- CH2 group
- CH3 group
- A longer hydrocarbon
Properties of Formate Esters
Common Characteristics
- Distinct odor
- Volatile
- React readily with water in hydrolysis
- Used in synthesis processes
Role of Water (H2O) in Organic Reactions
Water plays a major part in many transformations.
Why Water Is Reactive
Two Key Properties
- Polar molecule
- Can donate or accept protons (acid–base behavior)
Water in Ester Hydrolysis
Mechanism
- Water attacks the carbonyl carbon
- Forms an intermediate
- Breaks apart the ester
- Releases alcohol + acid
Reaction Mechanism Explained Simply
Hydrolysis involves a “splitting with water.”
Step 1: Nucleophilic Attack
Water molecule approaches carbonyl carbon.
Step 2: Tetrahedral Intermediate
A temporary structure forms.
Step 3: Bond Breaks
Ester linkage breaks apart.
Step 4: Products Form
The final result is:
- A carboxylic acid (HCOOH)
- An alcohol (CH₂OH-based)
Why Students Study This Reaction
Because it appears in:
- Exams
- Organic chemistry labs
- Mechanism practice
- Industrial chemistry examples
Importance in Real-World Chemistry
Hydrolysis of esters applies to many industries.
Pharmaceutical Industry
Reason
Drug molecules often contain ester groups that break down in the body.
Food Industry
Use
Flavor compounds are frequently esters.
Perfume and Fragrance Manufacturing
Role
Esters provide pleasant scents.
Polymer Industry
Example
Breakdown of polyester fibers involves hydrolysis.
Environmental Chemistry
Observation
Many natural decomposition processes involve water attacking ester groups.
Understanding CH2 in Chemical Structures
CH₂ is a basic building block of organic molecules.
Role of CH2
Functions
- Connects carbon frameworks
- Forms part of alcohol groups
- Serves as a backbone in hydrocarbons
Connection to the Formula
CH₂ likely connects the formate group to another functional group.
Learning the Formula Through Structural Representation
Visualizing the formula helps students understand its use.
Simple Structural Interpretation
H–C(=O)–O–CH₂ + H₂O
Breaking It Apart
Formate ester + water.
Final Products
Likely
- Formic acid
- Methanol or CH₂-based alcohol
Chemical Behavior of Formic Acid (HCOOH)
One product of hydrolysis is formic acid.
Characteristics
- Strong smell
- Simple carboxylic acid
- Used in preservatives and industrial chemicals
Methanol or CH₂ Alcohols
The other product may be a simple alcohol.
Uses
- Fuel additive
- Solvent
- Chemical feedstock
Why Formulas Like HCOOCH CH2 H2O Cause Confusion
Students often encounter incomplete formulas in assignments.
Common Reasons
- Condensed notation
- Missing bonds
- Context not provided
- Reaction implied rather than molecule shown
How to Interpret Ambiguous Formulas
Follow these steps:
Identify Functional Groups
Look for:
- Ester
- Alcohol
- Carboxyl group
Add Missing Hydrogens
Condensed formulas rarely show all atoms.
Consider Water’s Role
Water typically indicates hydrolysis or hydration.
Check Reaction Possibilities
Many confusing formulas represent a reaction, not a single molecule.
Uses of Formate Reactions in Education
Teachers use such formulas to teach:
Mechanisms
Understanding step-by-step reactions.
Structure Recognition
Identifying ester components.
Naming
Learning IUPAC rules.
Organic Logic
Determining what reacts with what.
Industry Applications of Formate Chemistry
Formate in Biofuels
Formates appear in clean-energy research.
Ester-Based Solvents
Used across industrial processes.
Agriculture
Formates used in feed preservation.
Chemical Synthesis
Serve as intermediates in organic reactions.
Safety Considerations
Even simple-looking formulas require proper handling.
Handling Formic Acid
Corrosive—use gloves.
Handling Methanol
Toxic—avoid ingestion or inhalation.
Laboratory Safety
Wear:
- Gloves
- Goggles
- Protective clothing
Environmental Impact of Hydrolysis Products
Chemists study how chemicals break down in nature.
Formic Acid
Biodegradable and occurs naturally.
Methanol
Breaks down quickly but toxic in high concentration.
Why Understanding HCOOCH CH2 H2O Matters
This formula helps students learn crucial concepts.
Teaches Reaction Mechanisms
Hydrolysis is foundational in organic chemistry.
Connects Theory to Real Life
Esters appear in food, fragrance, and pharmaceuticals.
Builds Structural Understanding
Recognizing functional groups is key to mastering organic chemistry.
Improves Chemical Interpretation Skills
Students learn to decode condensed formulas.
Conclusion
The formula HCOOCH CH₂ H₂O represents an important concept in organic chemistry—likely involving a formate ester undergoing hydrolysis in the presence of water. While the formula looks simplified or unusual, it captures the essence of how esters break down into acids and alcohols. Understanding this reaction is essential for chemistry students, researchers, and professionals working with organic compounds. By recognizing functional groups, reaction behavior, and structural relationships, learners gain a deeper appreciation for the transformative role water plays in chemical processes. Hydrolysis reactions like this one form the backbone of many natural, industrial, and biological systems.